What Is The Shelf Life Of A Ganache Cake?
Introduction to the use of technological sugars in ganaches.
Balancing technological sugars in ganaches
Sucrose molecule © Karl Harrison 3DChem.com
Having a basic understanding of sugars and how they interact in ganaches is an of import piece of noesis every chocolatier should delve into. Chocolatiers often overestimate the shelf life of their bonbons. In hotels and restaurants, this post is probably a little bit less of import, but even in that case, I would urge you to go on reading as some of the properties of the different sugars I'thousand covering hither will also employ to pastry preparations and can amend their quality, gustatory modality and shelf life. We often talk most 'sugars' in ganaches. The belatedly Jean Pierre Wybauw recommends a minimum 'sugar' content of 25% while Ramon Morato goes even farther and recommends a minimum of thirty% to attain a balanced recipe with optimal texture and shelf life. It'due south important to annotation that we talk here near 'sugars' as a group and not sucrose, commonly known every bit 'caster sugar' or 'granulated sugar' which is more specifically a disaccharide consisting of glucose and fructose molecules. To avoid confusion, I will only refer to this sugar as sucrose in this article. Sucrose is probably one of the worst sugars to use in ganache, to understand this nosotros need to wait at the country of saccharide at room temperature: it'south a coarse crystal. Considering of this Sucrose will, over time, re-crystallise and release h2o in the recipe leaving you with a reduced shelf life and subpar texture.
Why y'all demand to include sugars in your ganache
A ganache is an emulsion; meaning information technology's a mixture of h2o and fat. As h2o is what most microorganisms need to thrive, we need to "bind" this water to make it unavailable for those microorganisms to multiply. The value of "jump" versus "unbound" water is reflected by the so-called 'H2o Activity' or simply put 'AW.' A higher value equates to a college corporeality of unbound water, which ways the circumstances are more beneficial for microbial growth. A h2o activity measurement of 1 ways there is 100% of complimentary h2o, a measure out of 0.v ways at that place is 50% of free water.
| Water activity measurement | Shelf life |
| >0.85 | Shelf life of the ganache is less than 2 weeks |
| 0.85 – 0.70 | Shelf life of the ganache is between ii weeks and six weeks |
| 0.seventy – 0.65 | Shelf life of the ganache is between 6 weeks and iii months |
| <0.65 | Ganache is stable and shelf life exceeds six months |
When we wait at a ganache that is made up of just cream and chocolate, the score is usually well over 0.90. If you want to be able to plan your production and not produce your entire collection every other day, you will take to find a way to reduce the water activeness. Sugars are your chief tool to achieve this. Adding sure acids also help to lower the chances of microbial growth, but this will not exist suitable for every flavour profile. I'thousand likewise not a fan of resorting to booze equally you will effectively cutting out i-fifth of the global population as potential buyers of your production.
Which sugars are suitable
Then if we tin can't use adept old sucrose in our ganaches, which 'sugars' tin we use? Typically a combination of different sugars will do the task, to brand a decision we there are a few things we need to know.
Sweetening power
If we consider our ganache volition comprise anywhere betwixt 25 to 35% of sugars. The sweetening power of the sugars we will use volition be of critical consideration; afterwards all, we don't want overly sweetness chocolates. To understand this, we consider sucrose the benchmark, which has a sweetening ability of '100'. In the beneath tabular array I'm limiting myself to sugars we will typically use in chocolate, ice foam or pastry.
| Saccharide | Sweetening power | Structure |
| Sucrose | 100 | Disaccharide |
| Dextrose | 70 | Monosaccharide |
| Glucose syrup | 74 | Monosaccharide |
| Sorbitol | 50 | Sugar booze |
| Glycerol | threescore | Saccharide alcohol |
| Maltitol | 90 | Sugar booze |
| Invert sugar | 120 | Combination of fructose and glucose. |
If we expect at the above relative sweetening powers of the different sugars, and nosotros go along in listen nosotros need to reach a total of thirty% of 'sugars' (including the sucrose which is already present in the couverture we will be using) we want to avoid using besides many sugars which have a high relative sweetness. Peculiarly when we need to achieve a longer shelf life and need to increase the saccharide content fifty-fifty more, this becomes important. Relying solely on invert sugar for example will issue in a product that is too sweet, combining with a combination of sorbitol, glucose and or glycerol is the ameliorate way to go for creating the optimal residue between shelf life, counterbalanced sweet and optimal texture. Below I will break down several dissimilar sugars that I often utilize in my chocolates.
Glucose
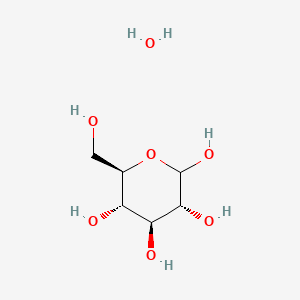
Glucose molecule
Benefits of glucose in chocolate are:
- Binds water thus reduces free h2o content. (lowers water activity)
- Prevents re-crystallisation of sucrose in the recipe.
- Makes a more than elastic ganache.
- Slows down the drying of the ganache.
Because glucose prevents re-crystallisation of sucrose, I recommend to always add a small amount in your recipes for this reason alone. Glucose syrup contains water, and and so that will need to exist deemed for in the full h2o content of the recipe. The amount of water is referred to in 'Beaumé', and typically glucose syrups come in the following forms: 43 BE = lxxx% dry substance 45 BE = 85% dry substance For ganaches, it'south brash to opt for 45BE as you will end upwards adding less h2o to the recipe. Another bespeak to continue in mind is the 'Dextrose Equivalent' abbreviated to DE. Dextrose is the production of hydrolysis of starch. The farther the hydrolysis process proceeds, the more reducing sugars are produced, and the higher the DE. Typically we find glucose syrups varying from 42 DE to 63 DE in industrial settings. The lower the DE, the higher the amount of dextrin and the lower the amount of reducing sugars. College DE glucose syrups have low amounts of dextrin and loftier quantities of reducing sugars. Notation:
- Dextrins are low-molecular-weight carbohydrates produced by the hydrolysis of starch or glycogen.
- Examples of reducing sugars are: glucose, fructose, lactose and maltose or just put monosaccharides.
- Glucose syrups with a college DE and thus a high amount of reducing sugars are sweeter.
For shelf life optimisation it is recommended to utilize 45DE glucose syrup, it contains a skilful amount of reducing sugars which are more effective at bounden water and preventing drying out of the ganache. A lower DE glucose syrup will brand the syrup more than viscose. Note: while glucose certainly has a identify in a ganache it is relatively ineffective compared to other sugars in reducing water action, to have a long shelf life glucose syrup should only be one element in the arsenal of water activity lowering ingredients in the ganache.
Invert sugar (commonly known as Trimoline)
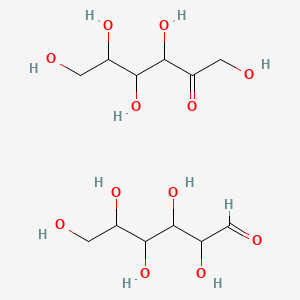
Fructose & Glucose molecule
Invert sugar is sucrose inverted by the enzyme 'invertase' and so becomes a mix of glucose and fructose (approximately a 50/50 ratio). Invert saccharide is particularly hygroscopic (water-attracting) and therefor an excellent addition to any ganache to make certain the ganache doesn't dry out. In doing this, the water activity volition also drib significantly. Honey is virtually identical to invert sugar, and they tin be substituted with each other. A few notes of caution:
- Excessive use of capsize saccharide will make the ganache mucilaginous and soft, which could be a significant issue for chocolates that will be enrobed.
- It's advisable not to heat capsize carbohydrate over 70 degrees C as fructose will showtime to degrade at this point, resulting in a loss of water activity lowering backdrop.
- Invert saccharide is quite sweet (sweetening ability of 125%), and then become piece of cake on information technology.
Sorbitol
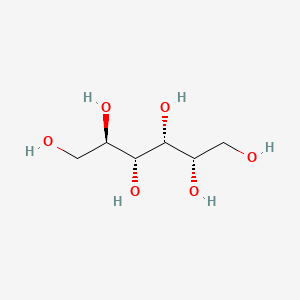
Sorbitol molecule
Sorbitol has a bad rep. For being an 'East-number'. Let me set the record straight though; sorbitol is a naturally occurring 'carbohydrate alcohol' and tin can be establish in grapes, pears and many other fruits! It is true that in excessive amounts, sorbitol can accept laxative effects, it would accept some severe chocolate-eating yet, to get anywhere near the necessary dose for that. Sorbitol has quite strong h2o activity lowering properties while only boasting 50% of the sweetness sucrose has, because of this information technology can be used in relatively larger quantities compared to invert carbohydrate without the cease product becoming overly sweetness. The recommended dose of Sorbitol is five – 10% calculated on your recipe.
Glycerol

glycerol molecule
Another ane with a bad rep. For like reasons to sorbitol. Like sorbitol, glycerol is a naturally occurring sugar booze. Some chefs claim that they don't similar the texture, but I would claiming anyone to gustatory modality several chocolates blindfolded and point out which ones have glycerol in them… I'll be impressed if anyone can approximate them all correctly. (if the ganaches were balanced correctly of course.) Glycerol has the most potent water activity lowering backdrop of any sugar we have discussed in this article, if a long shelf life is a must (think beyond 4 months without freezing) I find information technology challenging to attain this without the inclusion of glycerol. Key points of glycerol:
- Glycerol is approx. 2 times stronger than sorbitol at bounden water or lowering water action.
- The dose is 3 – five %, exceeding 12% volition outcome in a bitter unpleasant afterward taste.
- Glycerol has a sweetening ability of 60, so quite a chip lower than sucrose.
- Glycerol is a sugar that is naturally occurring in our bodies amongst other places.
Applying this in our recipes to get an optimal shelf life
Ramon Morato recommends that for optimal shelf life, a ganache should be roughly made upwardly similar this: Max. water content: twenty% sugars content +/- xxx% Cocoa butter +/- 21% Dairy fatty +/- 15% A real-life example of such ganache would be:
Cream 340g Capsize saccharide 90g Glucose 40g Sorbitol 100g 65% chocolate 620g Anhydrous butter 140g
Or broken down more than:
| Weight | % | |
| Total | one,330g | 100% |
| Sugar content | 441g | 33% |
| Fat content Of which cocoa butter Of which milk fat | 507g 248g 259g | 38% 18% 20% |
| Water content | 228g | 17% |
| Other dry substance | 155g | 12% |
To get to this calculation, nosotros need keep in mind: Cream (UHT) contains 35% dairy fat and 65% water 65% couverture contains 35% sucrose and xl% cocoa butter Glucose syrup contains 85% dry substance and fifteen% water.
Contrary to what you would probably expect, this ganache made under normal circumstances would have a water activeness reading of about 0.78, and then in optimal weather condition just a shelf life of about 6 weeks. As you tin can see if you want to exceed 8 weeks nosotros will need to consider the inclusion of glycerol.
Note: When shelf life is not essential, we can increment the water content to our liking (within reason), In commercial chocolate store settings still it is vital to follow the guidelines outlined in this article. People tend to overestimate significantly their shelf life, which could ultimately atomic number 82 to expensive lawsuits, don't permit it go to that! 😉 Update 02/07/2019: Since last writing this blog postal service, I took a masterclass with Alexandre Bourdeaux, one of the leading Barry Callebaut chefs and technical advisors, on shelf life. During those days, I learned that he broadly agrees with the ratios outlined in this post but to brand things easier for us chefs to calculate our recipes faster he created a software called Ganache solution, I was sceptical on its use merely later being shown how easy it is and how much time it tin can save I invested in this software, I would highly recommend professional chocolatiers to use this software. Note: The properties of these sugars extend beyond the world of chocolate bonbons. Including a % of invert carbohydrate in baked goods, for example, will proceed the product moist longer and thus increase shelf life. If you would like to learn more about this topic, 2 books have helped me a lot in understanding this topic better: One is the drove 'Fine chocolates' by J.P. Wybauw or the book 'Chocolate' by Ramon Morato.
'Chocolate' by Ramon Morato

Fine Chocolates drove by J.P. Wybauw
[/av_textblock]
© Arcane Chocolates – All-time Irish Chocolates
Share This Story, Choose Your Platform!
Title
What Is The Shelf Life Of A Ganache Cake?,
Source: https://arcanechocolate.com/ganache-formulation/
Posted by: wedelyoust1985.blogspot.com


0 Response to "What Is The Shelf Life Of A Ganache Cake?"
Post a Comment